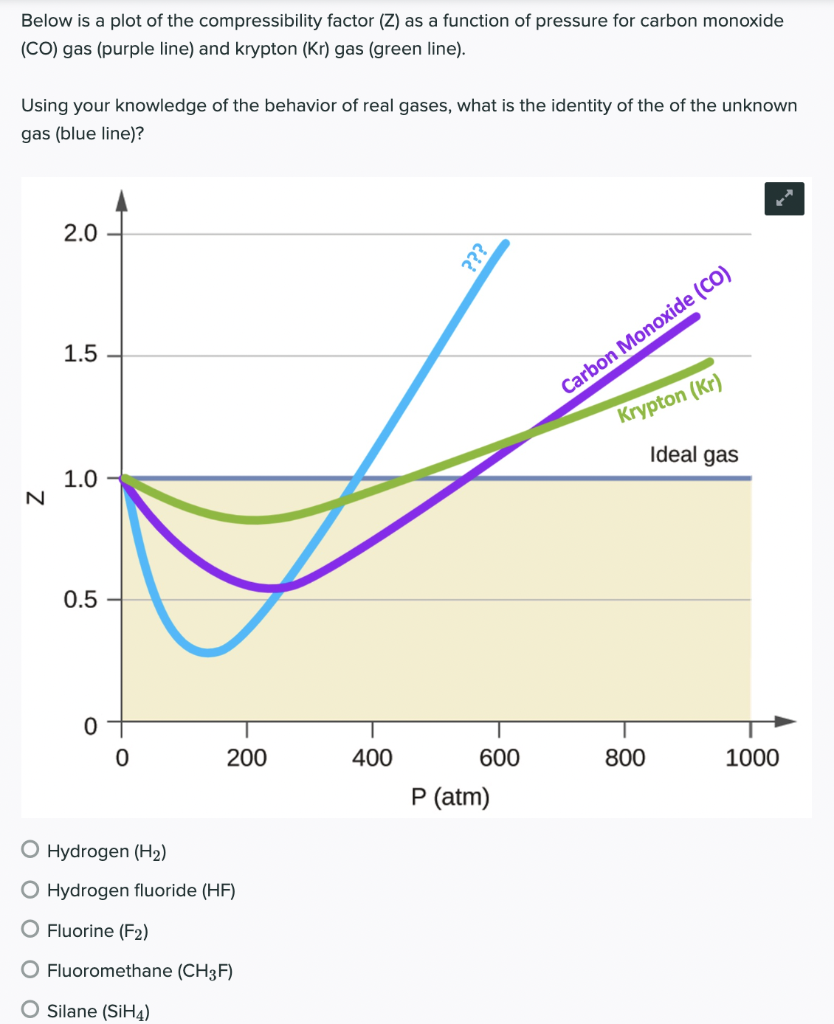
Compressibility factor Z = PV / nRT is plotted against pressure as
$ 22.99 · 4.8 (749) · In stock

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2
Solved The graph of compressibility factor (Z)v/sP for 1 mol

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure
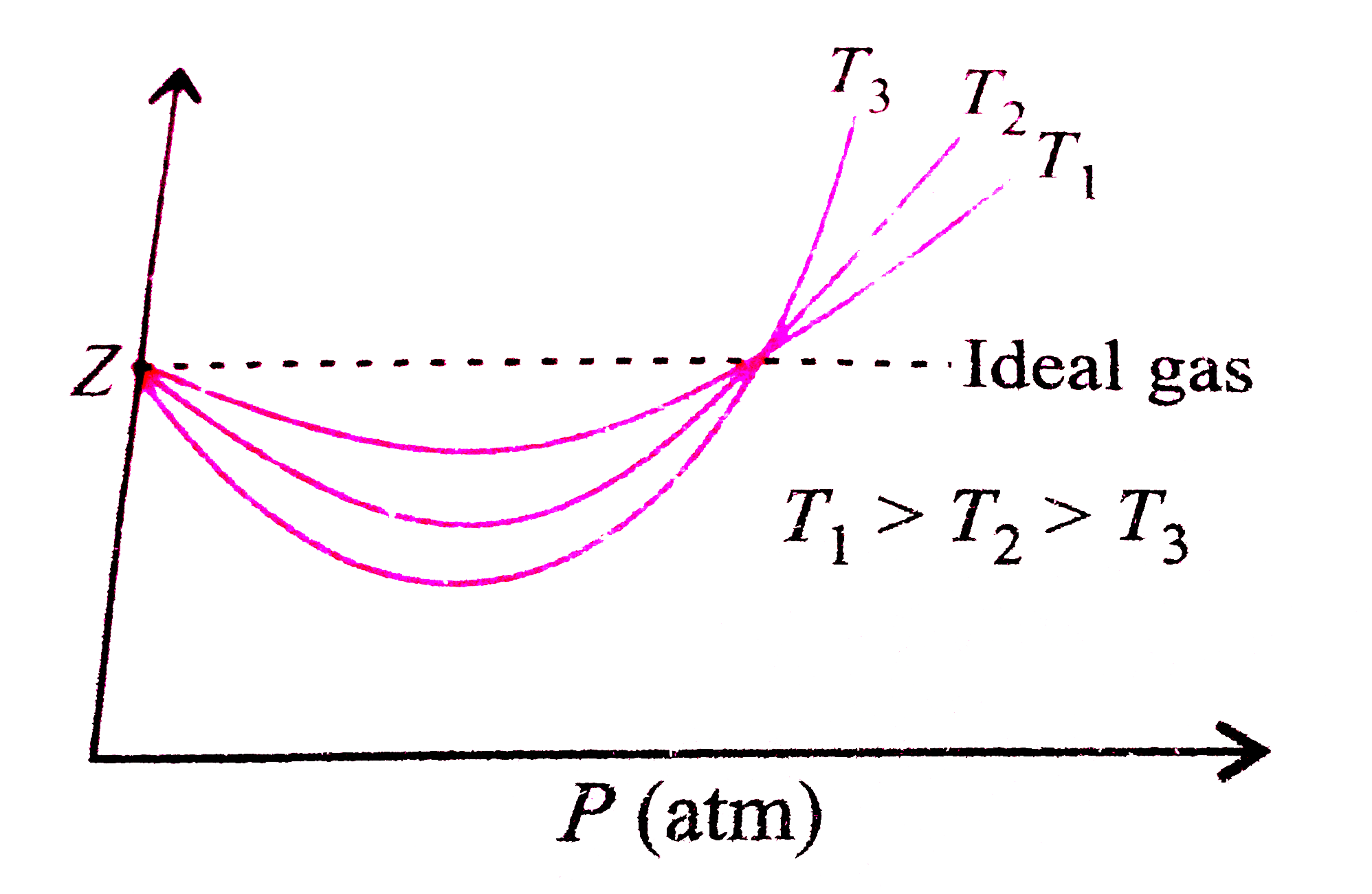
Consider a graph between compressibility factor Z and pressure P

PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H
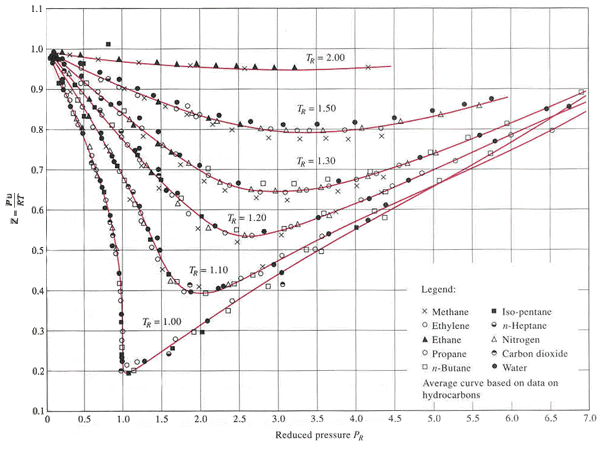
Untitled Document

Non-Ideal Gas Behavior Chemistry: Atoms First

Compressibility factor - Wikipedia

Chapter 5 Gases
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics
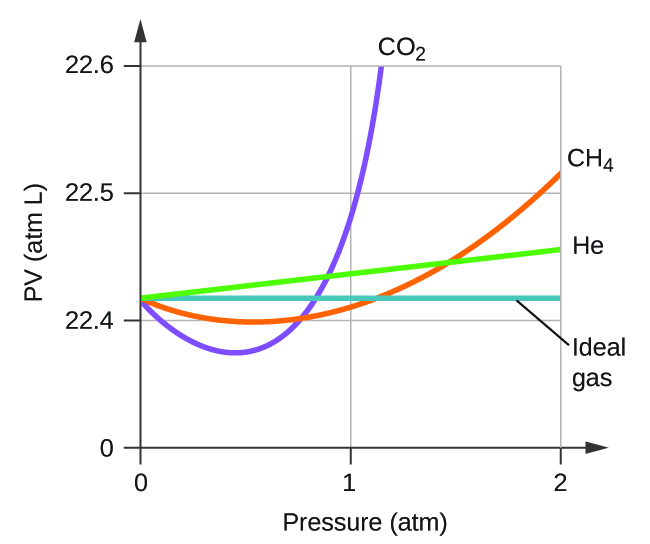
Non-Ideal Gas Behavior – Chemistry
Solved Below is a plot of the compressibility factor (Z) as

Compressibility factor - Wikipedia