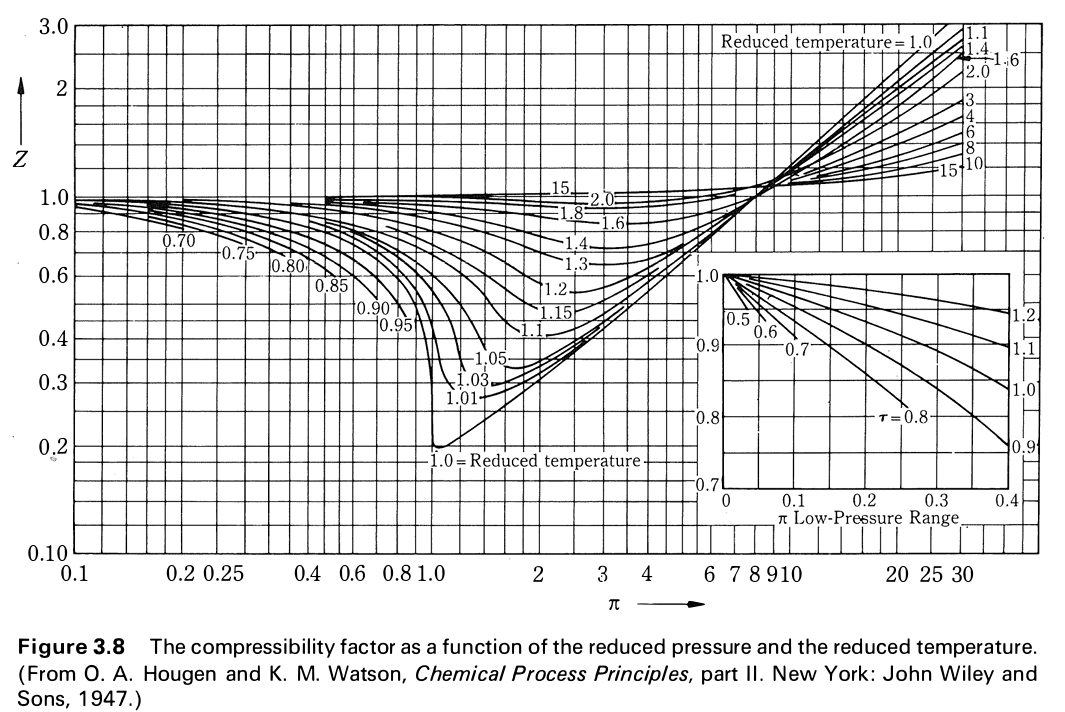
In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar
$ 15.99 · 4.9 (676) · In stock

Click here:point_up_2:to get an answer to your question :writing_hand:in the following compressibility factor z vs pressure graph at 300 k the compressibility of
Click here👆to get an answer to your question ✍️ In the following compressibility factor -Z- vs- pressure graph 300 K- the compressibility of CH-4- pressure - 200 bar deviates from ideal behaviour becauseThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is same as that in its ideal stateIntermolecular interactions between CH-4- molecules decreases

Chem-Eng-Musings Ideas Concepts Formula's Data

The following graph is plotted between compressibility factor Z versus pressure of a gas at different temperatures.Which of the following statements is /are correct?

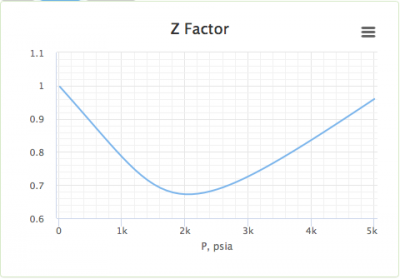
The graph of compressibility factor Z vs P for one mole of a real gas is shown in following diagram. The graph is plotted at a constant temperature 273 K. If the

Compressibility Factor Calculator - File Exchange - MATLAB Central

New explicit correlation for the compressibility factor of natural gas: linearized z-factor isotherms
Solved Use the graph of compressibility factors in terms of

WPILARIVIANN ZU 60. ollowing compressibility factor (2) vs pressure graph 300 K, the compresability of Cheatre 200 bar deviates from ideal behaviour because Compressibility Factor (2) Ideal gas 02 0 200 600

Acentric Factor - an overview
Chapter 03 - States of Matter - Module, PDF, Gases
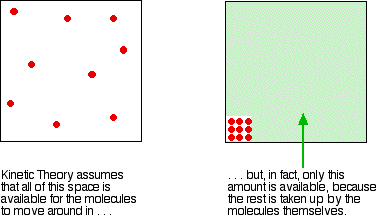
Real gases

Compressibility factor - Wikipedia