In the following compressibility factor (Z) vs. pressure graph 300
$ 17.50 · 4.6 (358) · In stock

Click here:point_up_2:to get an answer to your question :writing_hand:in the following compressibility factor z vs pressure graph at 300 k the compressibility of
Click here👆to get an answer to your question ✍️ In the following compressibility factor -Z- vs- pressure graph 300 K- the compressibility of CH-4- pressure - 200 bar deviates from ideal behaviour becauseThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is same as that in its ideal stateIntermolecular interactions between CH-4- molecules decreases
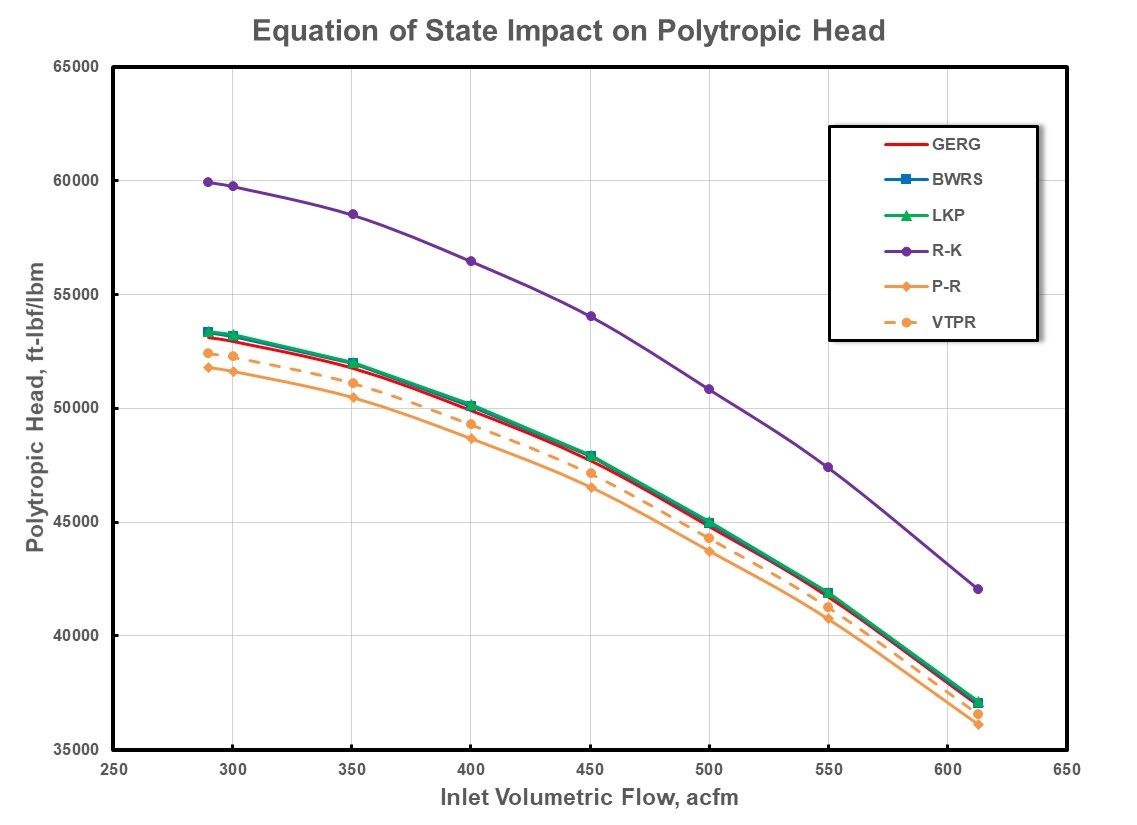
Compressor performance and thermodynamics
Which gas shows the maximum deviation from ideal gas, CO2 or NH3? Why? - Quora
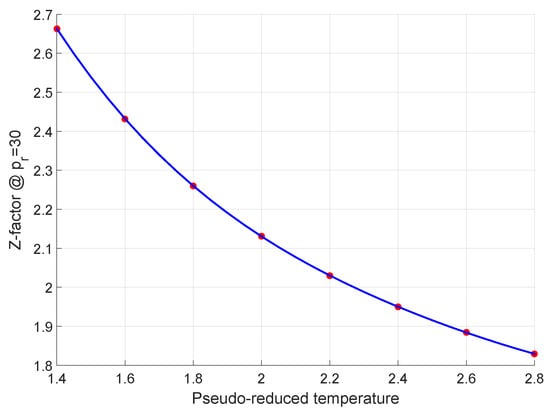
Energies, Free Full-Text

physical chemistry - Compressibility Factor Graph - Which gas attains a deeper minimum? - Chemistry Stack Exchange

47. In the following compressibility factor (Z) vs pressure graph 300 K, the compressibility factor of CH4 pressures < 200 bar deviates from ideal behavior because

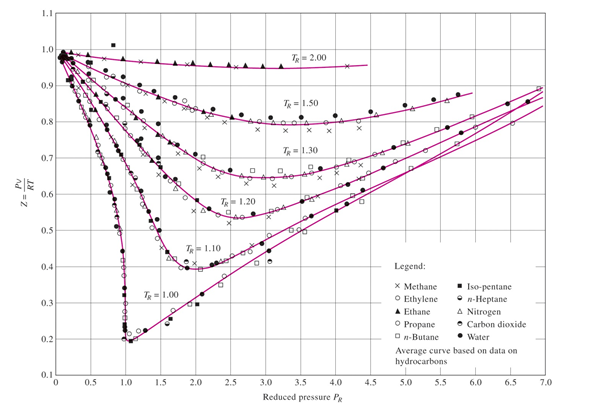
The role of the compressibility factor Z in describing the volumetric behavior of gases

Compressibility factor of water vapor along its saturation curve. Error

WPILARIVIANN ZU 60. ollowing compressibility factor (2) vs pressure graph 300 K, the compresability of Cheatre 200 bar deviates from ideal behaviour because Compressibility Factor (2) Ideal gas 02 0 200 600

Compressibility Charts McGraw-Hill Education - Access Engineering

If the slope of 'Z' (compressibility factor) vs. 'p' curve is constant `(slope=(pi)/(492.6)atm^
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora

Chemistry Desk: Effect of Pressure
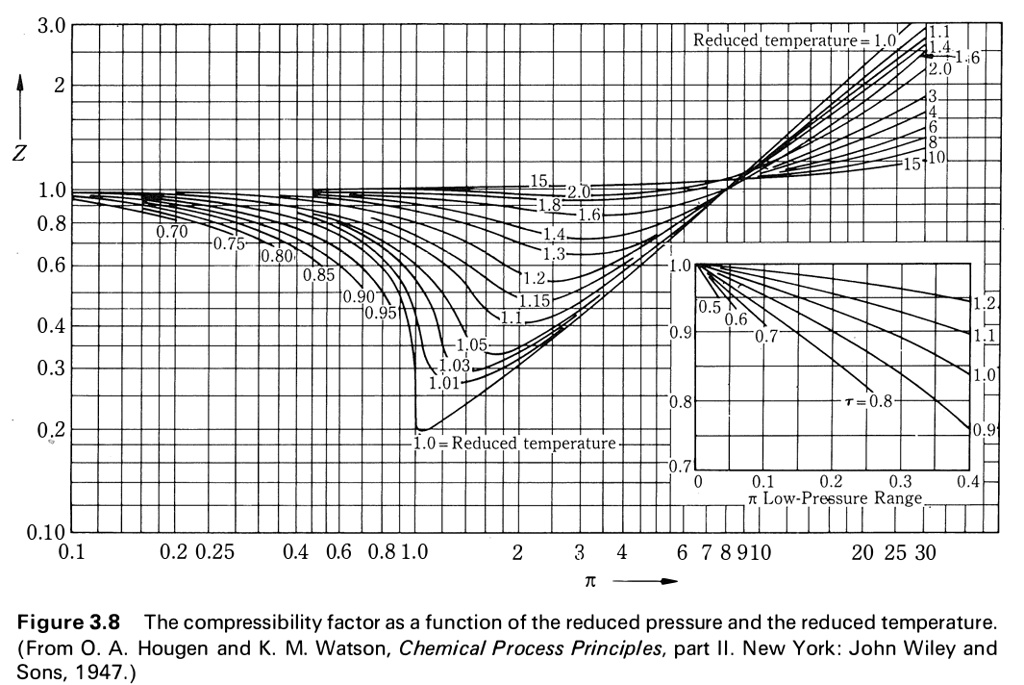
SOLVED: Use the graph of compressibility factors in terms of reduced variables to answer the following about methane (pc = 4.595 MPa, Tc = 190.55 K, and M = 16.04 g/mol): a)