physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
$ 18.99 · 4.8 (716) · In stock

The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In

gas laws - Graph of compressibility factor vs pressure when real
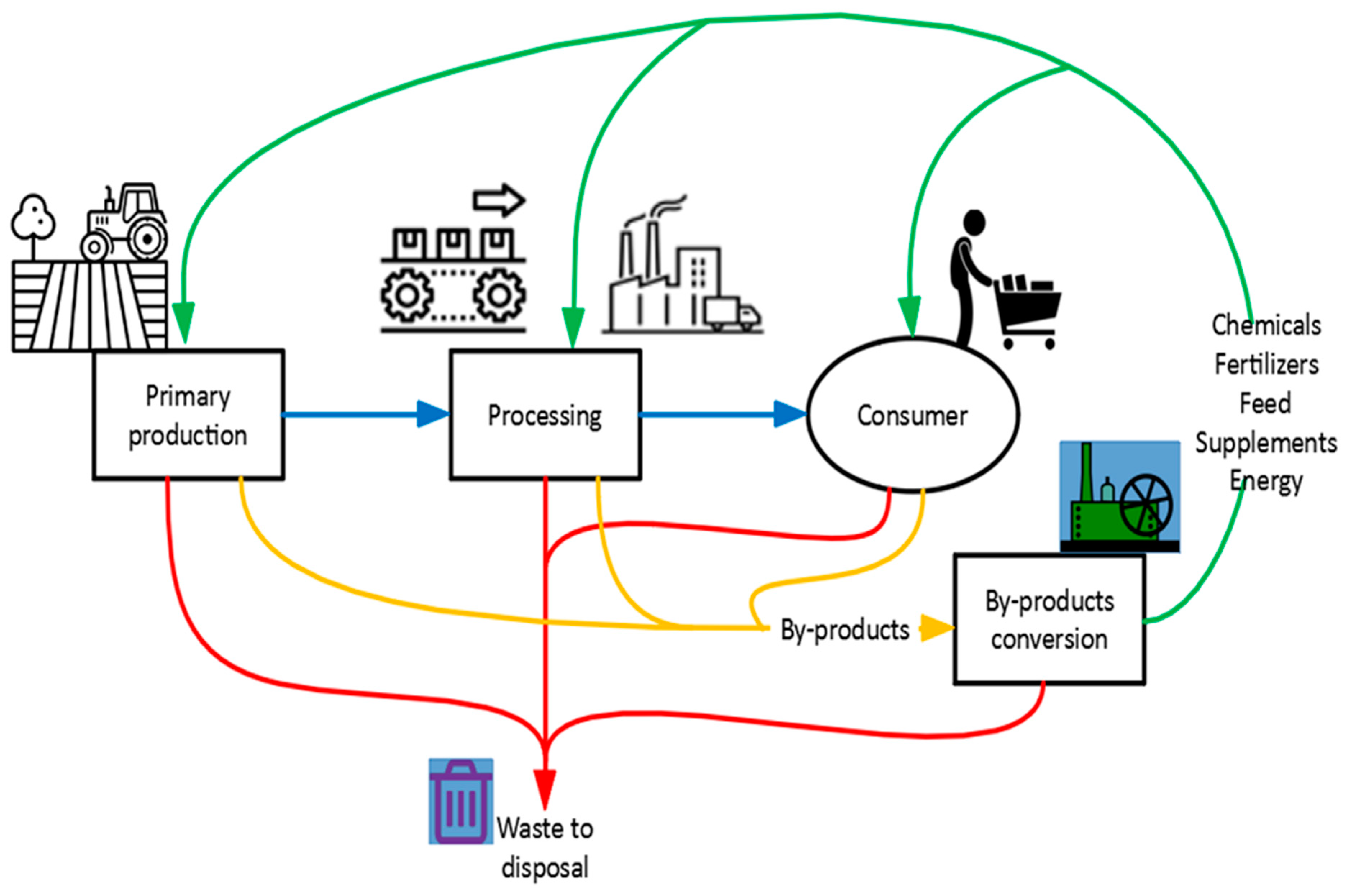
Energies, Free Full-Text
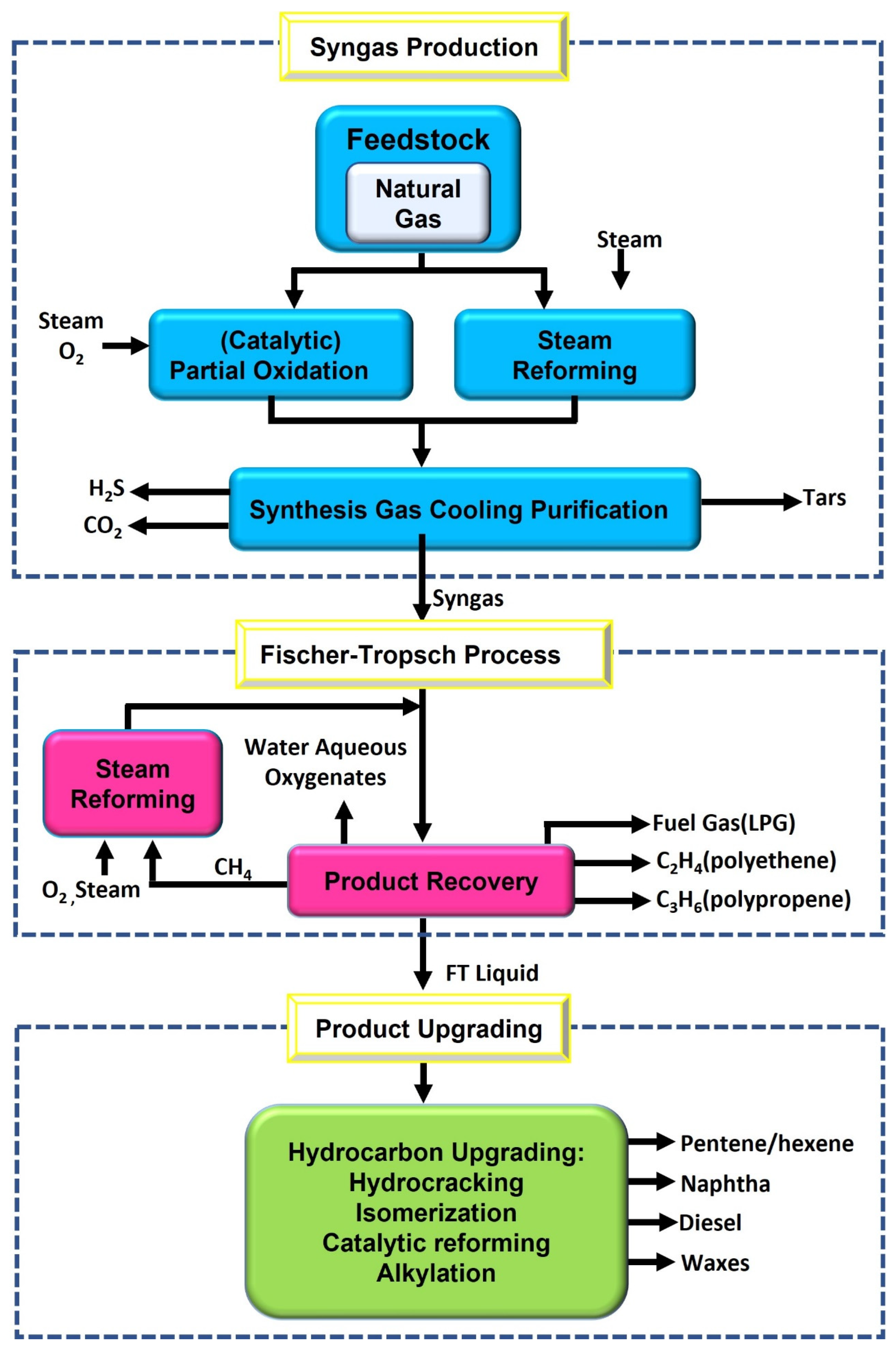
Methane, Free Full-Text

Non-Newtonian Flow to the Theoretical Strength of Glasses via
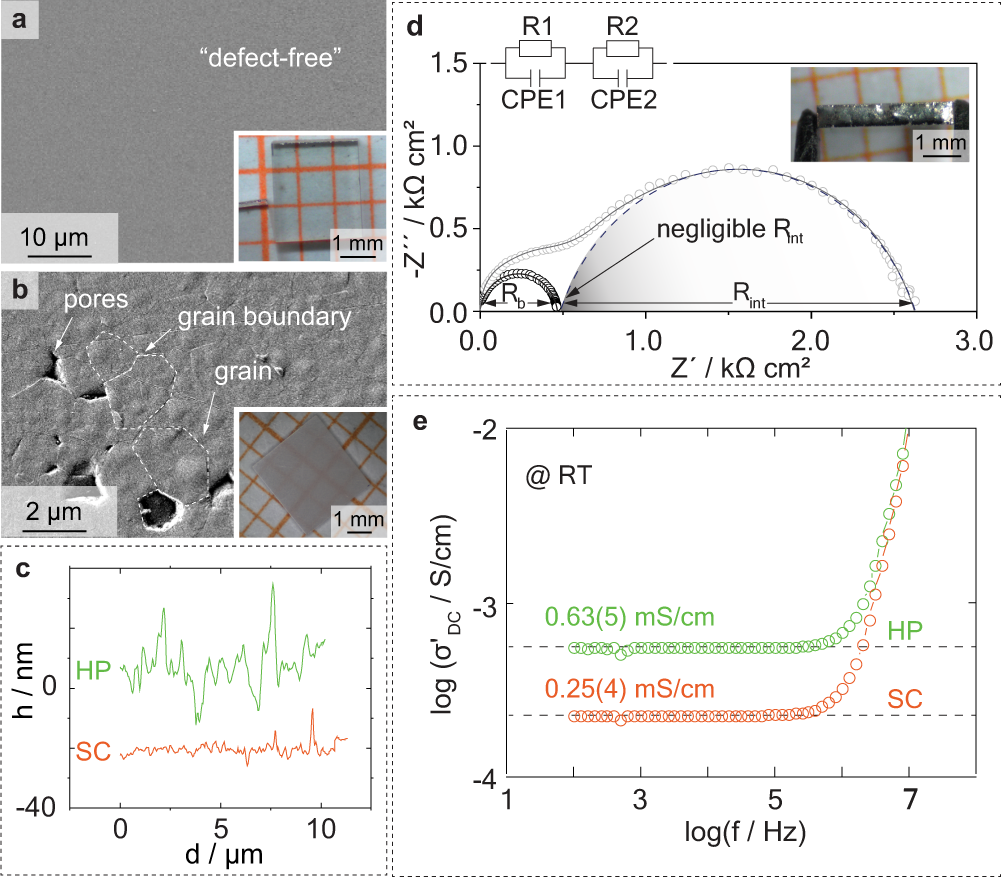
Effect of pulse-current-based protocols on the lithium dendrite
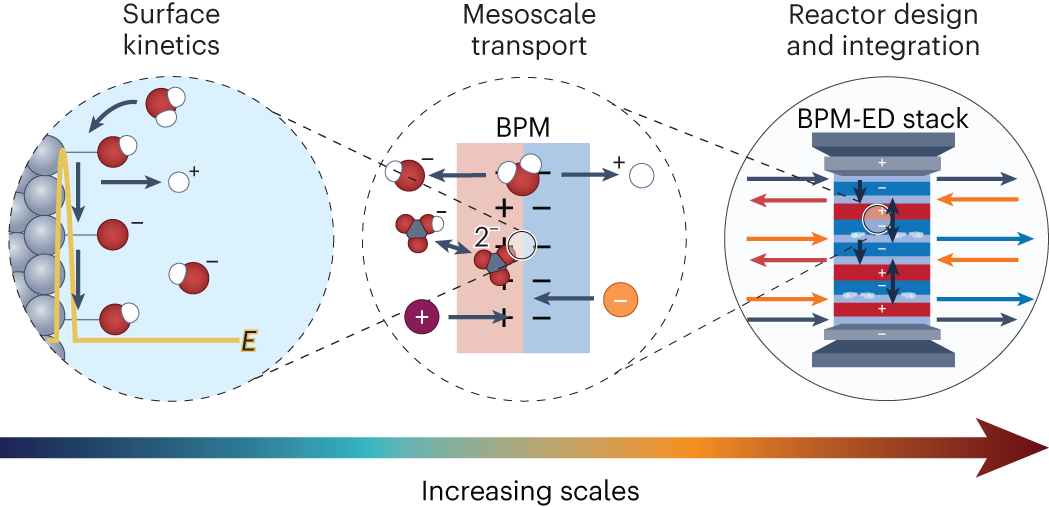
Multi-scale physics of bipolar membranes in electrochemical
822 questions with answers in PHYSICAL CHEMISTRY
Why compressibility factor of areal gas is greater than unity at

Description of real gases: Compression factor
VISIONS OF A HYDROGENECONOMY - THE ELEMENT HYDROGEN

Compressibility factor (gases) - Citizendium

Ideal gas - Wikipedia
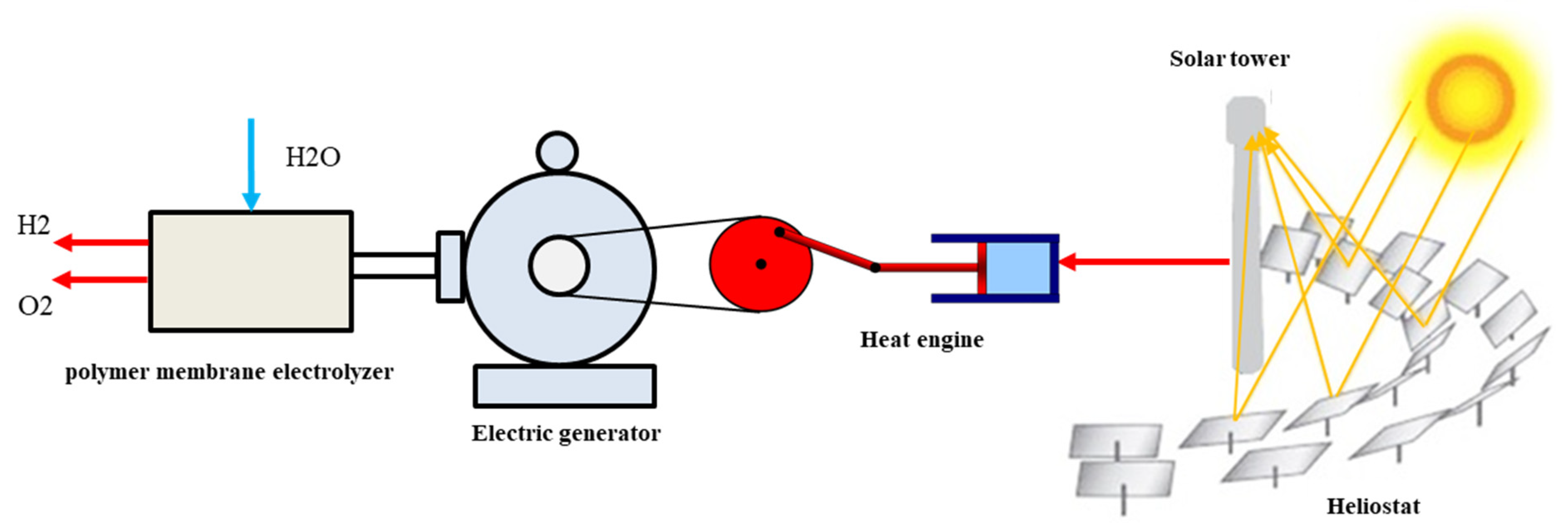
Molecules, Free Full-Text









